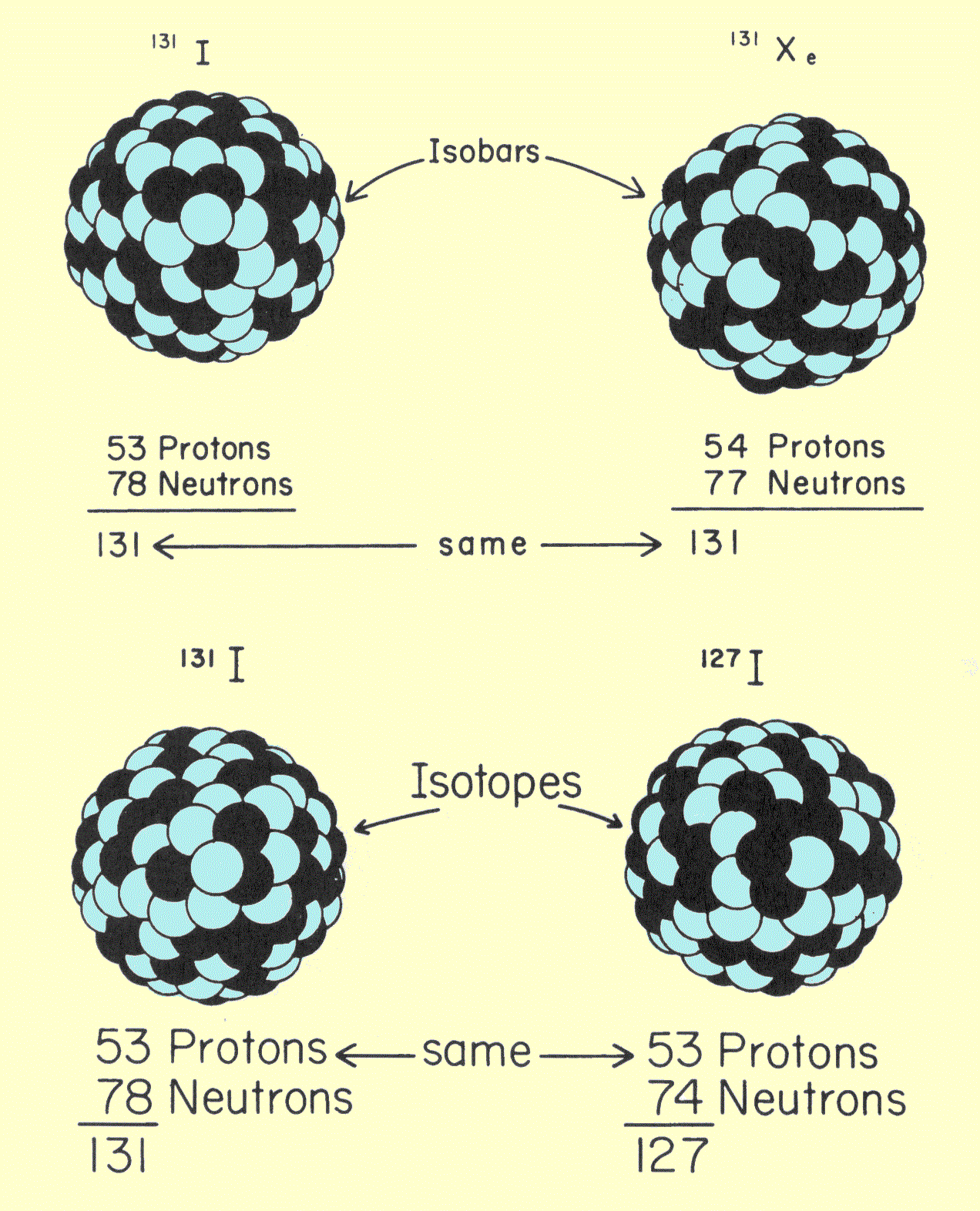
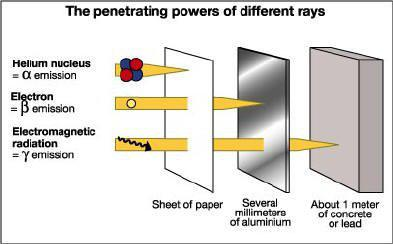
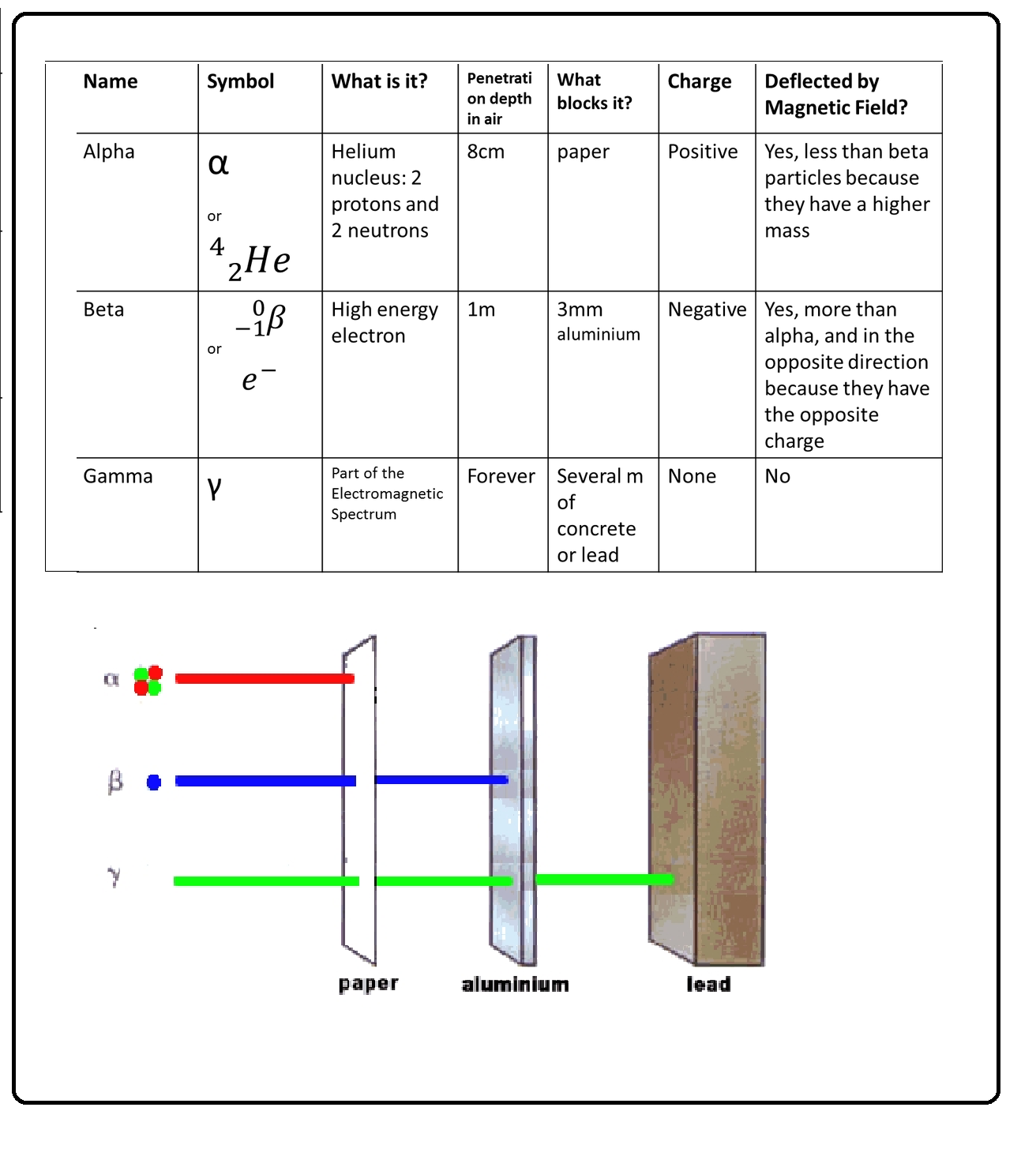
Q 2612545439
 Calculate the number of protons, neutrons and electrons in `text()_(35)^(80)Br`
Calculate the number of protons, neutrons and electrons in `text()_(35)^(80)Br`


Solution:
In this case `text()_(35)^(80)Br , Z = 35 , A = 80, ` species is neutral
Number of protons = number of electrons
` = Z = 35`
Number of neutrons `= 80 – 35 = 45,`
In this case `text()_(35)^(80)Br , Z = 35 , A = 80, ` species is neutral
Number of protons = number of electrons
` = Z = 35`
Number of neutrons `= 80 – 35 = 45,`